The Issue
Food safety is recognized as a universal public health concern. Enormous resources are invested globally to ensure that there is a safe food supply. However, most of these resources are focused on improving food safety through the reduction of harm from chemical and microbial contaminants present in foods, rather than on the safety of the foods themselves.
What We Are Doing
The Agriculture & Food Systems Institute addresses this challenge by providing information resources and capacity building activities for scientists and regulators, focusing particularly on the safety assessment of foods and feeds derived from genetically engineered plants.
Why It’s Important
Evidence-based food safety assessments conducted using internationally accepted best practices help reduce complexity, time, and costs for all stakeholders. They also contribute to regulatory and trade cooperation between countries.
Program Staff

Karen Hokanson, Ph.D.

Bhavneet Bajaj, Ph.D., PMP
Collaborators & Partners
- Asia-Pacific Economic Cooperation High Level Policy Dialogue on Agricultural Biotechnology (APEC HLPDAB)
- African Union Development Agency New Partnership for Africa's Development (AUDA NEPAD)
- Biotech Consortium India Limited
- Charles River Laboratories
- China Center for Disease Control and Prevention
- China Ministry of Agriculture and Rural Affairs
- Chinese National Institute for Nutrition and Health
- Chinese Society of Agricultural Biotechnology
- Corteva Agrisciences (formerly DuPont Pioneer)
- Indonesian National Agency for Food and Drug Control (BPOM)
- Indonesian Center for Animal Research and Development (ICARD)
- Institute for International Crop Improvement, Donald Danforth Plant Science Center
- Inter-American Institute for Cooperation on Agriculture (IICA)
- Rutgers University
- Stine Haskell Research Center
- Thailand Department of Agriculture
- USDA Foreign Agricultural Service
- U.S. Environmental Protection Agency
- U.S. Food and Drug Administration
Programs

Sharing Knowledge & Expertise
Understanding the need for technical capacity building for food safety assessment, the Agriculture & Food Systems Institute shares its knowledge and scientific expertise by facilitating regional training workshops and developing and disseminating reference materials.
LEARN MORE
Understanding Crop Composition as a Basis for Comparative Assessment
Our Crop Composition Database is a curated, open access resource that provides data on the natural variability in the nutrients, anti-nutrients, and secondary metabolites of key crop species. This information is critical to inform comparative assessments of genetically engineered foods and feeds, but it can also inform research that promotes the healthy growth of livestock, as well as improving food security and nutrition modeling.
VISIT DATABASE
Providing Risk Assessors with Centralized Food Safety Information
The Agriculture & Food Systems Institute has prepared a series of monographs that provide comprehensive reviews of publicly available food safety information and data for the novel proteins found in some genetically engineered plants.
LEARN MOREOur Successes
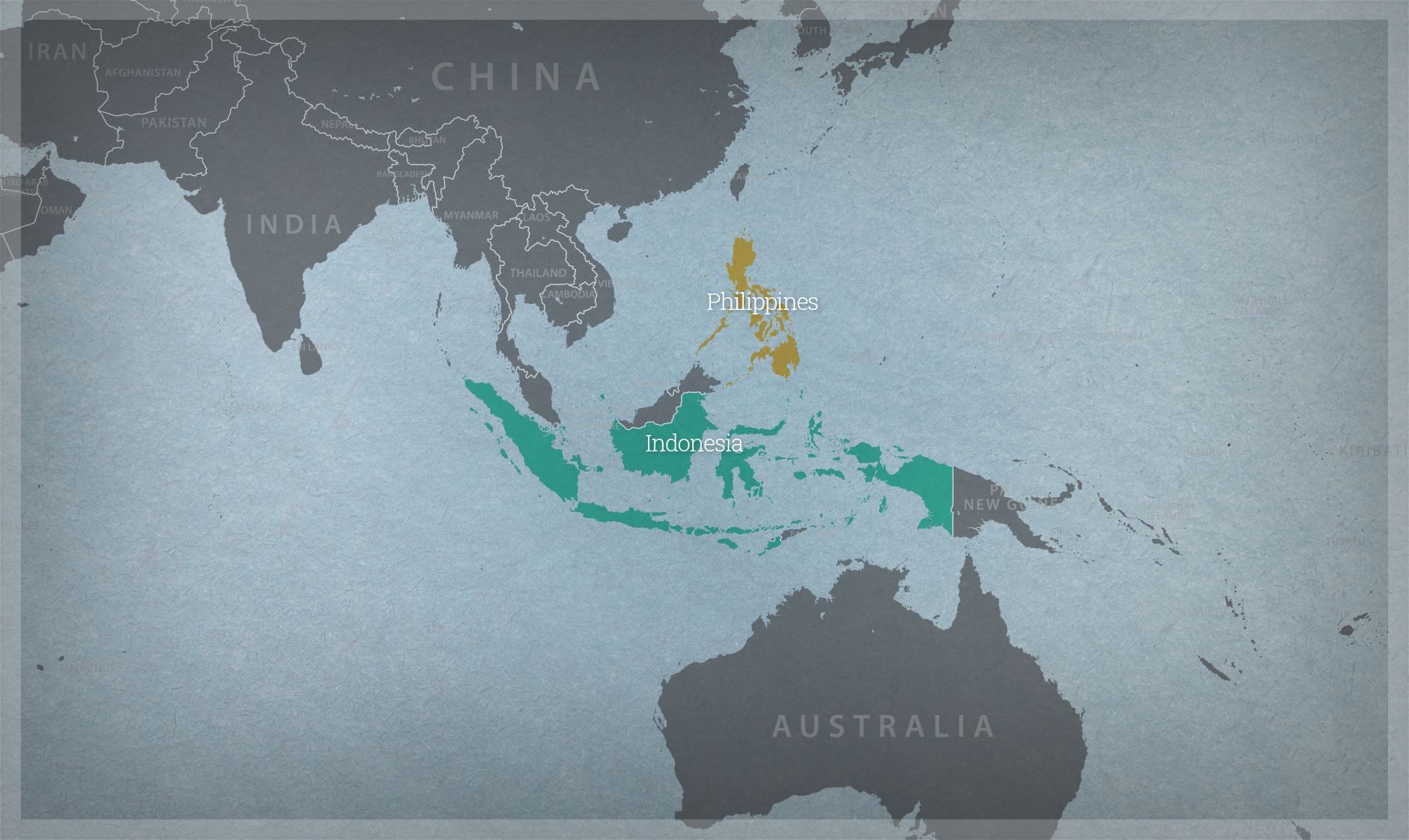
Sharing updates on biotechnology and the status of biosafety policies contributes to increasing awareness of the potential benefits of agricultural biotechnology for food security and climate resiliency. The Association of Southeast Asian Nations (ASEAN) Secretariat, with support from the United States Department of Agriculture (USDA) and the Department of State, sought to explore the potential for increased regulatory harmonization, streamlined approval processes, and adoption of effective and efficient regulatory approaches.
Hosted by ASEAN and organized by the Agriculture & Food Systems Institute on March 4-6, 2024, the workshop on “Bolstering ASEAN Food Security through Partnership and Cooperation on Agricultural Biotechnology” brought together delegates from 11 ASEAN member and observer states, as well as stakeholders from international organizations and the private and public research sector, to discuss regulatory practices for products derived from modern biotechnologies and opportunities for regulatory cooperation among ASEAN countries. At the workshop:
- Expert speakers from Canada, Australia, the African Union, and the United States provided insights into the global status of biotechnology and biotechnology products in international trade, as well as examples of efforts for bilateral and multilateral cooperation and harmonization, to increase awareness of the potential benefits of agricultural biotechnology for food security and climate resiliency.
- A representative from each ASEAN member state presented the status of their countries’ biotechnology and biosafety policies, with additional presentations from the Philippines and Singapore on the status of their policies for new breeding techniques.
- The participants traveled to Bandung, where they visited a farm producing seed of the genetically engineered late blight resistant potato approved in Indonesia, followed by the University of Padjadjaran, where there is ongoing research on genetically engineered catfish.
- The delegates and participants concluded the workshop with facilitated discussions on opportunities for future collaboration and harmonization in line with ASEAN’s mission.
-
Project Name:
Bolstering ASEAN Food Security through Partnership and Cooperation on Agricultural Biotechnology
-
Year:
2024
-
Host:
Association of Southeast Asian Nations (ASEAN) Secretariat
-
Funding:
United States Department of Agriculture (USDA) and Department of State
-
ASEAN Member States:
Brunei, Cambodia, Indonesia, Lao PDR, Malaysia, Myanmar, Philippines, Singapore, Thailand, Timor-Leste, Vietnam
Early career researchers are empowered by opportunities to share their research on a global stage. To provide young scientists with an international platform to highlight innovations in the field of agricultural biotechnology, the United States Department of Agriculture and Agriculture & Food Systems Institute co-organized the “Early Career and Innovative Start-ups Symposium” in Seattle on July 29, 2023 to enrich discussions at the Asia-Pacific Economic Cooperation High Level Policy Dialogue on Agricultural Biotechnology (APEC HLPDAB) plenary.
The symposium provided 16 early career researchers and developers from a dozen APEC member economies with the opportunity to deliver lightning talks and poster presentations on their agricultural biotechnology research, encouraging them to become advocates for the development of trade-facilitating, science-based policies on agricultural biotechnology. Additionally, industry representatives delivered presentations on enabling policy environments, current research and development, and career opportunities. As one of the activities on the margins of the APEC HLPDAB plenary, the symposium, by highlighting innovations in the field, contributed to strengthening sustained information sharing related to agricultural biotechnologies between APEC member economies.
-
Project Name:
Early Career and Innovative Start-ups Symposium
-
Year:
2023
-
Partner:
United States Department of Agriculture Foreign Agricultural Services New Technologies and Production Methods Division (USDA FAS NTPMD)
-
Participant Member Economies:
Australia, Canada, Chile, Chinese Taipei, Indonesia, Japan, Republic of Korea, Malaysia, Philippines, Thailand, USA, and Vietnam
The safety assessment of genetically engineered foods is heavily informed by internationally agreed guidelines developed under the Codex Alimentarius Commission, including Principles for the Risk Analysis of Foods Derived from Modern Biotechnology and the Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Plants. Together, these documents describe a safety assessment process for whole foods, rather than food additives or ingredients that have traditionally been the subject of safety assessments. To support the practical application of these guidelines, the Agriculture & Food Systems Institute developed a comprehensive training program for delivering experiential learning to regulators from across the globe.
The program, which covers the key elements used in genetically engineered food safety assessment, was developed by AFSI scientific staff in collaboration with internationally recognized subject matter experts, in response to needs identified by:
- Regulators and scientists from the public sector who are called upon to conduct safety assessments
- Public sector scientists engaged in product development
- Scientists from public institutions that provide testing services
The multi-phased program helps participants:
- Establish a baseline understanding of the concepts and principles of genetically engineered food safety assessment (Phase I)
- Observe and understand acute and sub-chronic toxicity testing protocols, reporting formats, and results interpretation (Phase II)
The program combined introductory e-learning modules, classroom training, and practical exercises (Phase I) with laboratory visits, discussions with the technicians and supervisors who generate safety data, and observation of testing and data collection (Phase II). Participants gained a deep understanding of the rationale behind the safety assessment framework, the practical realities of laboratory testing, the context needed to understand and interpret the data they see as part of regulatory submissions, and how to better interpret and understand published literature.
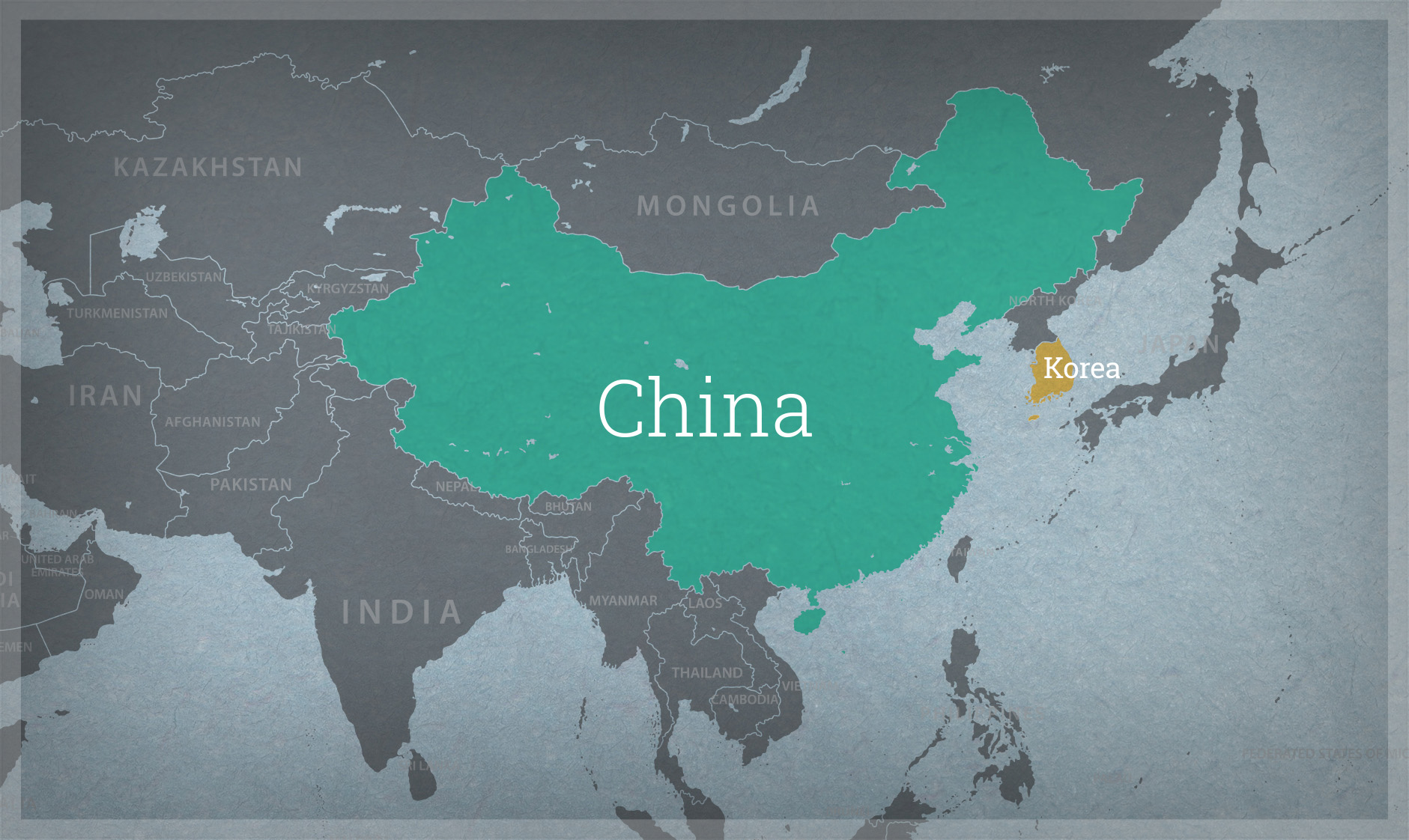
The program has been conducted three times since 2014, providing technical training to over 50 participants from 9 countries.
- 2014: Bangladesh, Ghana, India, Kenya, Nigeria, Pakistan, and Paraguay
- 2017: Indonesia
- 2019: China
A “refresher” training program planned in cooperation with the Government of Indonesia to take place in 2020 was turned into a virtual training course (Phase III), featuring fully-voiced interactive training sessions prepared by AFSI staff and food safety experts from Australia, the Philippines, and the United States. It revisits the comprehensive food safety assessment paradigm presented in Phases I and II and expands these concepts into considerations for plants containing multiple genetically engineered traits. The training was capped off by an interactive session with expert faculty so that participants could ask follow-up questions and provide program feedback.
-
Project Name:
Comprehensive GE Food Safety Assessment Training for Foods Derived from GE Plants
-
Years:
2014-2020
-
Partners:
Biotech Consortium India Limited, Charles River Laboratories, China Center for Disease Control and Prevention, China Ministry of Agriculture and Rural Affairs, Chinese National Institute for Nutrition and Health, Chinese Society of Agricultural Biotechnology, Corteva Agrisciences (formerly DuPont Pioneer), CropLife China, Estel Consult Ltd., ILSI Focal Point in China, Indonesian National Agency for Food and Drug Control (BPOM), Indonesian Center for Animal Research and Development (ICARD), Institute for International Crop Improvement, Donald Danforth Plant Science Center, Rutgers University, Stine Haskell Research Center, United States Department of Agriculture Foreign Agricultural Service (USDA FAS), United States Environmental Protection Agency (EPA), United States Food and Drug Administration (FDA)
-
Countries:
Bangladesh, China, Ghana, India, Indonesia, Kenya, Nigeria, Pakistan, and Paraguay
Discover
Publications
Towards a Harmonised Approach to Food Safety Assessment of Genetically Engineered Plants in South Asia – Expert Working Group Report
December 20, 2021
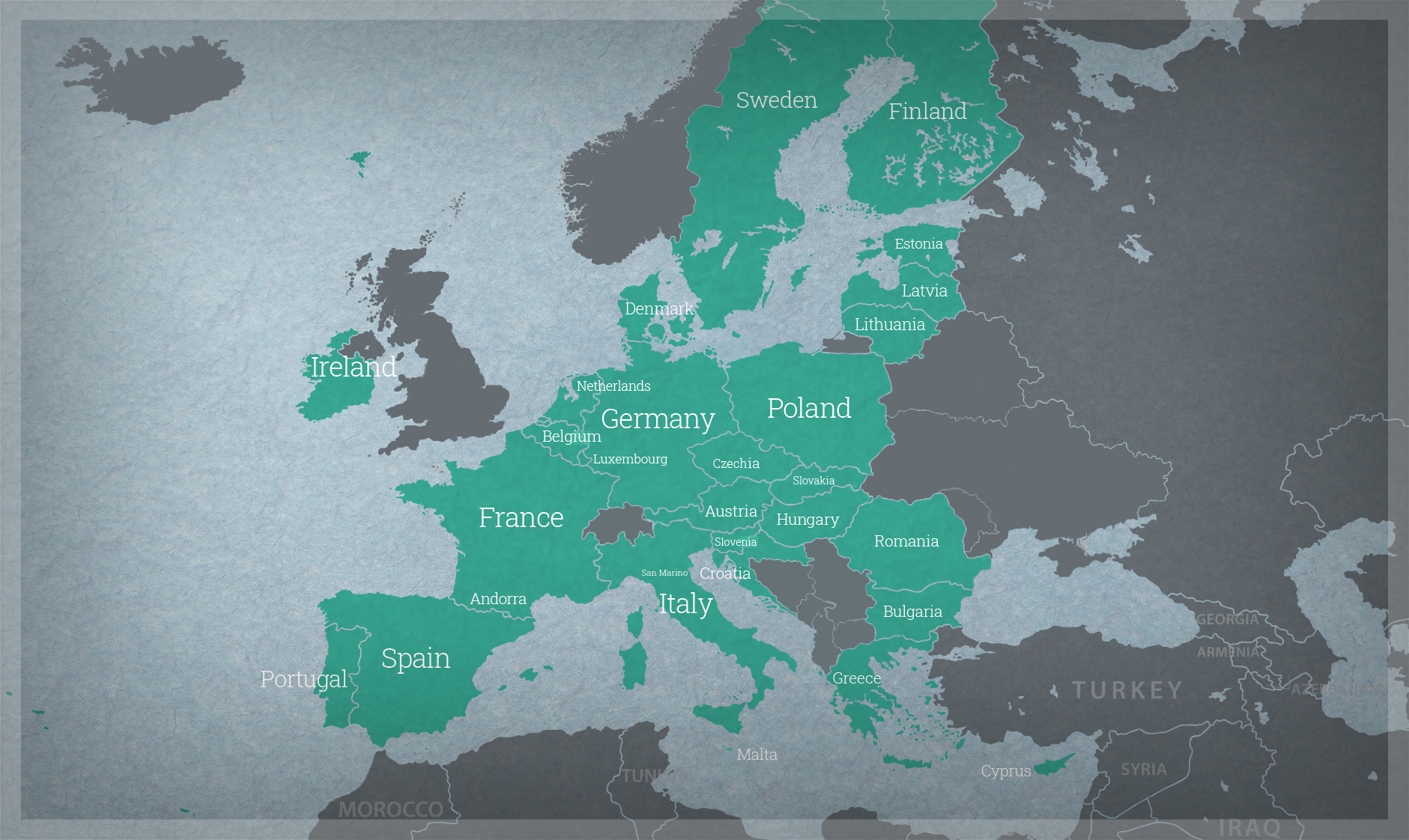
The harmonisation initiative in South Asia was formally undertaken in 2020 by the Agriculture & Food Systems Institute (AFSI) by convening an Expert Working Group (EWG) constituted of senior experts and regulators identified from agencies in Bangladesh, Bhutan, India, and Sri Lanka that are relevant to the safety assessment of foods derived from rDNA plants. This report was systematically drafted by the EWG.
Low Level Presence in Seed: A Science Based Approach to Expedited Environmental Risk Assessment
March 31, 2014
The Agriculture & Food Systems Institute organized a workshop held in Buenos Aires, Argentina on December 18-19, 2013 on low level presence (LLP) in seed. This document contains the conference proceedings to address the potential adverse environmental impacts that might arise from an LLP in seed situation using a consistent and scientifically defensible approach to environmental risk assessment.
The Use of Whole Food Animal Studies in the Safety Assessment of Genetically Modified Crops: Limitations and Recommendations
Critical Reviews in Toxicology | January 1, 2013
This manuscript by Bartholomaeus et al., published in Critical Reviews in Toxicology, focuses on the relevance and utility of whole food animal studies in safety assessments of GM crops (Task Force #10).
Natural Variation in Grain Composition of Wheat and Related Cereals
Journal of Agricultural and Food Chemistry | January 1, 2013
Proceedings from the 2012 IFBiC Plant Compositional Analysis Workshop, published in the Journal of Agricultural and Food Chemistry (Shewry et al. 2013;61(35):8295-8303) (Task Force #12).
How Composition Methods Are Developed and Validated
Journal of Agricultural and Food Chemistry | January 1, 2013
Proceedings from the 2012 IFBiC Plant Compositional Analysis Workshop, published in the Journal of Agricultural and Food Chemistry (Rogers. 2013;61(35):8312-8316) (Task Force #12).
Bringing a Transgenic Crop to Market: Where Compositional Analysis Fits
Journal of Agricultural and Food Chemistry | January 1, 2013
Proceedings from the 2012 IFBiC Plant Compositional Analysis Workshop, published in the Journal of Agricultural and Food Chemistry (Privalle et al. 2013;61(35):8260-8266) (Task Force #12).
Application of Laws, Policies, and Guidance From the United States and Canada to the Regulation of Food and Feed Derived From Genetically Modified Crops: Interpretation of Composition Data
Journal of Agricultural and Food Chemistry | January 1, 2013
Proceedings from the 2012 IFBiC Plant Compositional Analysis Workshop, published in the Journal of Agricultural and Food Chemistry (Price and Underhill. 2013;61(35):8349-8355) (Task Force #12).
Biological Importance and Statistical Significance
Journal of Agricultural and Food Chemistry | January 1, 2013
Proceedings from the 2012 IFBiC Plant Compositional Analysis Workshop, published in the Journal of Agricultural and Food Chemistry (Lovell. 2013;61(35):8340–8348) (Task Force #12).
Availability and Utility of Crop Composition Data
Journal of Agricultural and Food Chemistry | January 1, 2013
Proceedings from the 2012 IFBiC Plant Compositional Analysis Workshop, published in the Journal of Agricultural and Food Chemistry (KItta. 2013;61(35):8304–8311) (Task Force #12).
Food Safety: Importance of Composition for Assessing Genetically Modified Cassava (Manihot esculenta Crantz)
Journal of Agricultural and Food Chemistry | January 1, 2013
Proceedings from the 2012 IFBiC Plant Compositional Analysis Workshop, published in the Journal of Agricultural and Food Chemistry (Jansen van Rijssen et al. 2013;61(35):8333-8339) (Task Force #12).
Compositional Analysis of Genetically Modified (GM) Crops: Key Issues and Future Needs
Journal of Agricultural and Food Chemistry | January 1, 2013
Proceedings from the 2012 IFBiC Plant Compositional Analysis Workshop, published in the Journal of Agricultural and Food Chemistry (Hoekenga et al. 2013;61(35):8248–8253) (Task Force #12).
Evaluation of Endogenous Allergens for the Safety Evaluation of Genetically Engineered Food Crops: Review of Potential Risks, Test Methods, Examples and Relevance
Journal of Agricultural and Food Chemistry | January 1, 2013
Proceedings from the 2012 IFBiC Plant Compositional Analysis Workshop, published in the Journal of Agricultural and Food Chemistry (Goodman et al. 2013;61(35):8317–8332).
Events
-
 Second Capacity Building Workshop on Food Safety Assessment of Genetically Engineered Plants
Second Capacity Building Workshop on Food Safety Assessment of Genetically Engineered PlantsJune 3, 2025-June 4, 2025
New Delhi, India
-

-
 OECD Working Party on the Safety of Novel Foods and Feeds (WP-SNFF)
OECD Working Party on the Safety of Novel Foods and Feeds (WP-SNFF)March 26, 2025-March 28, 2025
Paris, France
-
 HLPDAB: Fourth Consultative Meeting on Regulatory Cooperation – Advancing the Policy Approaches Document
HLPDAB: Fourth Consultative Meeting on Regulatory Cooperation – Advancing the Policy Approaches DocumentJanuary 15, 2025
Virtual, Singapore