The Issue
Developing and applying sound science to the environmental risk assessment (ERA) of biotechnologies is crucial for safely realizing their contributions to human health and sustainable production of food, fuel, and fiber.
What We Are Doing
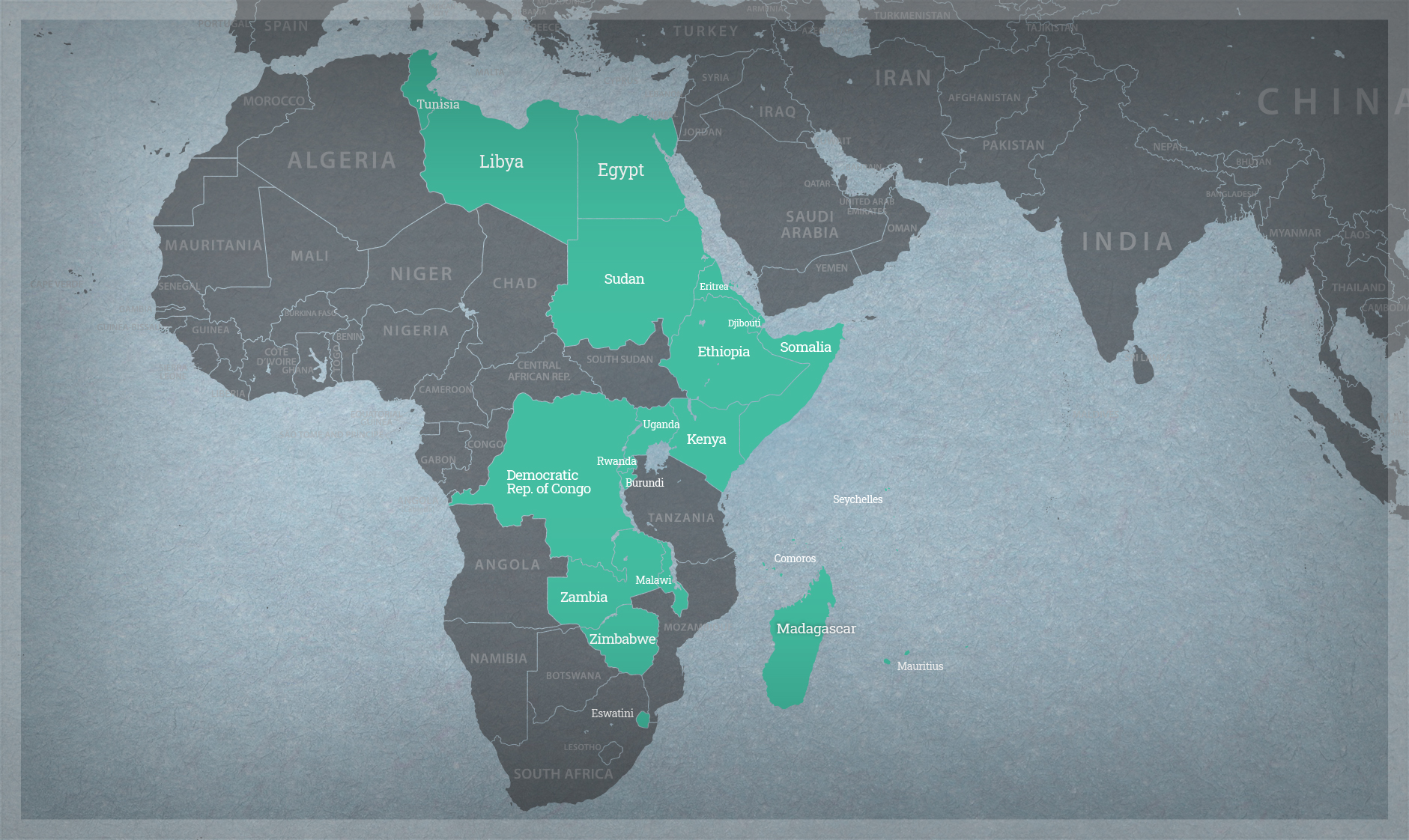
Whether it be through improving systematic approaches to inform understanding of plausible risks associated with the use of gene drive strategies or working to maximize the utility of confined field trial data, we serve as a scientific resource for governments, academic institutions, and private sector organizations.
Why It’s Important
Any new technology comes with potential benefits and potential risks for the environment. Understanding these is essential to making informed decisions about what should or should not move from research to field to market.
Program Staff

Andrew Roberts, Ph.D.

Karen Hokanson, Ph.D.
Collaborators & Partners
- Association for Strengthening Agricultural Research in Eastern and Central Africa (ASARECA)
- Australian Government
- Common Market for Eastern and Southern Africa (COMESA)
- Foundation for the National Institutes of Health (FNIH)
- International Food Policy Research Institute (IFPRI)
- Instituto Interamericano de Cooperación para la Agricultura (IICA)
- New Partnership for African Development (NEPAD)
- Organisation for Economic Co-operation and Development (OECD)
- University of British Columbia
- University of Edinburgh
- USDA Foreign Agricultural Service
Programs

Promoting Use of Relevant Field Trial Data Across Geographies
Confined field trials (CFTs) are conducted to inform environmental risk assessments of genetically engineered crops. But instead of repeating trials in every country where a genetically engineered crop might be grown, data from previous trials, including those cultivated in similar agroclimates, may provide all that is necessary.
LEARN MORE
Rationalizing the Location of CFTs to Maximize Data Utility
Our online tool allows users to visualize agroclimatic zonations and their relationships to CFT locations in order to help determine where to best locate CFTs and maximize data utility. This can be used by CFT managers and risk assessors to provide context for the relevance of CFT data produced at remote locations.
VISIT TOOL
Identifying Priorities for Risk Assessment
With almost 263 million estimated cases of malaria worldwide in 2023, novel approaches to managing this debilitating disease are needed. We are advancing understanding of the underlying technology and helping stakeholders identify priorities for risk assessment of the use of gene drive to control the mosquito vectors that spread this disease.
LEARN MOREOur Successes
Most regulatory systems have developed standard test methods and data requirements for addressing the potential effects on non-target organisms (NTO) resulting from their exposure to genetically engineered plants expressing Bt proteins (insecticidal proteins primarily derived from Bacillus thuringiensis). However, recent developments in multiple technologies have led to the production of genetically engineered plants that incorporate pest protection without pesticidal proteins (e.g., using RNAi) or the use of proteins that are not functionally related to Bt proteins. Although the overall assessment paradigm for genetically engineered plants is robust and can still be used to produce NTO assessments, a need was identified to explore the appropriate NTO tests and measurement endpoints for these new technologies.
With funding from the Agricultural Biotechnology Stewardship Technical Committee (ABSTC), the Agriculture and Food Systems Institute (AFSI) organized the Workshop on Sublethal Endpoints in Non-Target Organism (NTO) Testing for Non-Bt Genetically Engineered Crops on March 4-5, 2019 in Washington, DC. The meeting convened scientists from academia, government, and industry to review the progress and status of sublethal endpoint testing in non-target organisms and answer the following questions:
- What are the appropriate tests and measurement endpoints needed to inform non-target arthropod assessment for technologies that have a different mode of action than the Bt Cry proteins?
- When is it appropriate to conduct sublethal endpoint assessment testing?
- What types of assays should be employed?
Presentations and subsequent discussions at the workshop related to criteria for making decisions regarding sublethal endpoint assessment testing helped shape a manuscript titled “Sublethal Endpoints in Non-Target Organism Testing for Insect-Active GE Crops,” which was published in Frontiers in Bioengineering and Biotechnology on June 9, 2020. The paper:
- Provides an overview of the current status and history of sublethal endpoint use in insect-active genetically engineered crops.
- Evaluates the future use of sublethal endpoints for new and emerging technologies.
-
Project Name:
Sublethal Endpoints in Non-Target Organism Testing for Insect-Active Genetically Engineered Crops
-
Years:
2019-2020
-
Funding:
Agricultural Biotechnology Stewardship Technical Committee (ABSTC)
-
Parties:
Scientists from Academia, Government, and Industry
-
Geography:
USA
Because it is not possible to measure, monitor, and test every organism in every environment, surrogate species play an important role in evaluating the potential for adverse environmental impacts of new technologies. This includes assessing the potential effects of genetically engineered crops on non-target organisms (NTOs). Surrogate species must be selected appropriately to represent key taxonomic or functional groups, and testing must be done with sufficient rigor to ensure that tests address the potential impact being investigated. The Agriculture and Food Systems Institute (AFSI) recognized the benefits of providing technical training to regulatory scientists and risk assessors on non-target organism testing of transgenic crops.
AFSI facilitated multiple expert working groups and publications addressing the proper use of surrogate species for testing in support of environmental risk assessment of genetically engineered crops, and in 2016, the institute produced a peer-reviewed publication that provides an overview of the history of regulatory approaches to NTO assessments and analyzes current regulatory practices. The paper proposes recommendations to improve the predictive value and efficiency of NTO testing and to facilitate the transportability of existing datasets across geographies and regulatory regimes.
Supported by a grant from the United States Department of Agriculture Foreign Agricultural Service (USDA FAS) Emerging Markets Program (EMP) and in cooperation with the USDA Agricultural Research Service (ARS), Iowa State University, and Corteva Agriscience, AFSI organized technical trainings on non-target organism testing of transgenic crops for two different cohorts on June 26-30, 2023 and June 24-28, 2024. These by-invitation-only workshops provided regulatory scientists and environmental risk assessors from Ghana, India, Indonesia, Kenya, Malawi, Nigeria, the Philippines, the USA and Vietnam with an experiential learning opportunity in laboratory and field testing of non-target organisms.
During the technical training program, through group work, presentations, and facilitated exchanges, participants:
- Learned how insect populations are assessed in agroecosystems using two common insect collection techniques: sticky cards to collect flying insects and pitfall traps to collect ground-dwelling insects.
- Gained first-hand experience setting up traps in established fields of insect-resistant and conventional corn.
- Received the opportunity to review and critique data generated by commercial laboratories, which are typically provided to government regulators in support of applications to authorize commercial planting of insect-resistant crops.
- Improved their understanding of the limits of NTO testing in environmental risk assessment.
-
Project Name:
Environmental Risk Assessment Workshop: Non-Target Organism Testing
-
Years:
2023-2024
-
Funding:
United States Department of Agriculture Foreign Agricultural Service (USDA FAS) Emerging Markets Program (EMP)
-
Parties:
Regulatory Scientists, Environmental Risk Assessors
-
Geography:
Ghana, India, Indonesia, Kenya, Malawi, Nigeria, the Philippines, the USA and Vietnam
Genetically engineered Ae. aegypti have been developed to control populations of this mosquito, which is the main vector of yellow fever, dengue fever, Zika, and chikungunya. To facilitate risk assessments for the use of these genetically engineered mosquitoes, regulators need access to succinct, summary information on biosafety-relevant aspects of the biology of the species. Biosafety consensus documents published by the Organisation for Economic Co-operation and Development (OECD) Working Group on Harmonization of Regulatory Oversight in Biotechnology (WG-HROB) provide internationally agreed-upon baseline information for understanding key aspects of the biology of specific organisms. Each document identifies and describes important characteristics of its subject, usually a plant species, which are typically considered when undertaking a comparative risk assessment of its genetically engineered counterpart. The Agriculture and Food Systems Institute (AFSI) recognized the benefits of developing an OECD Consensus Document of the Biology of mosquito Aedes aegypti to serve as a public resource.
As a recognized observer organization and having consistently attended meetings for many years, AFSI approached the OECD Environment Secretariat about developing a biology document for Aedes aegypti. The idea was put to the WG-HROB, and, with the governments of Brazil and Mexico as convening leads together with AFSI, the project was launched at a workshop hosted by the government of Mexico in May 2014. During this workshop, experts developed a detailed outline of the document and committed to an action plan.
The OECD Consensus Document on the Biology of Mosquito Aedes aegypti was declassified in July 2018 as Volume 8 of the series on Safety Assessment of Transgenic Organisms in the Environment. It is the first OECD biosafety consensus document to:
- Deal with the biology of an insect.
- Focus on an organism for which biotechnological applications are not aimed at an increase in production or the quality of the product, but are driven by health considerations, specifically to reduce the health burden associated with diseases vectored by mosquitoes, such as yellow fever, dengue, Zika and Chikungunya.
The biology document provides the following information on Ae. aegypti:
- Taxonomy
- Morphology
- Life Cycle
- Reproductive Biology
- Genetics
- Ecology
- Interactions with Other Species and the Environment
Preparation of the OECD Consensus Document of the Biology of Mosquito Aedes aegypti was co-led by Mexico, Brazil, and AFSI, with additional expertise provided by Australia, France, India, Kenya, and the United States, as well as inputs from private sector entities.
-
Project Name:
OECD Consensus Document on the Biology of Mosquito Aedes Aegypti
-
Years:
2014-2018
-
Co-Leads:
Governments of Mexico and Brazil
-
Additional Expertise:
Governments of Australia, France, India, Kenya, and the United States, as well as experts from private sector entities
-
Publisher:
OECD Working Group on Harmonization of Regulatory Oversight in Biotechnology (WG-HROB)
Historically, genetically engineered (GE) plants incorporating genes conferring insect protection have primarily used Cry proteins derived from Bacillus thuringiensis (Bt) to achieve their insecticidal phenotype. As a result, regulators have developed familiarity and confidence in reviewing plants incorporating these insecticidal proteins. However, new technologies have been developed to produce genetically engineered plants that incorporate pest protection by triggering an RNA interference (RNAi) response or proteins other than Bt Cry proteins. With increasing attention being paid to the use of sublethal endpoints and their value for environmental risk assessment (ERA), the Agriculture and Food Systems Institute (AFSI) recognized a need to clarify the protection goals and information needs for the ERA of RNAi Plants.
To address this need, AFSI:
- Highlighted the benefits of clarifying protection goals and information needs for the ERA of RNAi Plants at the 2012 Entomological Society of America Annual Meeting, which took place on November 11-14, 2012, in Knoxville, Tennesee.
- Organized a scientific session on RNAi and environmental risk assessment of genetically engineered plants at the 13th International Symposium on the Biosafety of Genetically Modified Organisms (ISBGMO), which took place on November 9-13, 2014 in Cape Town, South Africa. During this event, a multi-sectoral, multi-disciplinary team of scientists worked together to help identify and clarify the relevant safety considerations and risk assessment methodologies for environmental risk assessment of plants with RNAi traits.
- Led a joint effort to draft a manuscript based on presentations and discussions at the 13th ISBGMO scientific session, which proposes research areas that may be beneficial for future environmental risk assessments of RNAi-based genetically modified plants, with a particular focus on non-target organism assessment. This peer-reviewed paper was published in Frontiers in Plant Science on November 10, 2015.
-
Project Name:
Biosafety Research for Non-Target Organism Risk Assessment of RNAi-based Genetically Engineered Plants
-
Years:
2012-2015
-
Funding:
United States Department of Agriculture National Institute for Food and Agriculture (USDA NIFA) Biotechnology Risk Assessment Grants (BRAG) Program
Discover
Publications
Frequently Asked Questions: Genome Edited Plants
Biosafety Resource Book Series | April 4, 2024
Published by the South Asia Biosafety Program, this booklet provides information about genome edited plants in a simple language, so readers may better understand the science, applications, and policies at the global and national levels.
GEnZ Explorer: A Tool for Visualizing Agroclimate to Inform Research and Regulatory Risk Assessment
Transgenic Research | June 6, 2023
This paper describes the development of an open-source tool, the GEnZ Explorer, that provides agroclimate together with overall crop production information to assist regulators and applicants in making informed choices on whether data from previous CFTs can inform an environmental risk assessment in a new country, as well as help developers determine optimal locations for planning future CFTs. The GEnZ Explorer allows users to identify the agroclimate zones that are relevant for the production of 21 major crops and crop categories.
New Applications of Insecticidal RNAi
Frontiers in Agronomy | May 27, 2022
Reduced reliance on synthetic chemical pesticides that may negatively impact pollinators and other beneficial insects is an aim of both private industry and governmental agencies alike. The need for environmentally sound methods of insect control has inspired research into a wide range of bio-based technologies. RNA interference has emerged as a highly effective tool with inherent potential to control a wide range of insects.
Frequently Asked Questions: Genetically Engineered Plants and Biosafety
Biosafety Resource Book Series | March 30, 2021
The South Asia Biosafety Program designed this booklet to address some of the questions the general public may have about the safety of genetically engineered plants, with explanations in easy and understandable language.
Biosafety Regulation and Processes in Bangladesh: A Guide for Researchers in Agricultural Biotechnology
Biosafety Resource Book Series | November 19, 2020
Part of the South Asia Biosafety Program’s capacity development interventions, this publication aims to inform researchers about the prevailing regulatory administrative system of Bangladesh and outlines the regulatory processes functioning at different stages of research and development of GE crops.
Paraguay’s Path Toward the Simplification of Procedures in the Approval of GE Crops
Frontiers in Bioengineering and Biotechnology | August 18, 2020
This paper presents the recent evolution of the regulatory system in Paraguay toward the establishment of a simplified procedure for GE crops that have been already assessed by sound and experienced regulatory systems, taking into account several scientific criteria. Dr. Carmen Vicién, Agriculture & Food Systems Institute in-country partner, was a co-author of this paper, which references the Agriculture & Food Systems Institute’s involvement in the Partnership for Biosafety Risk Assessment and Regulation in Paraguay.
Sublethal Endpoints in Non-Target Organism Testing for Insect-Active GE Crops
Frontiers in Bioengineering and Biotechnology | June 9, 2020
This review paper focuses on the current status and history of sublethal endpoint use in insect-active GE crops and evaluates the future use of sublethal endpoints for new and emerging technologies.
Genetic Biocontrol for Invasive Species
Frontiers in Bioengineering and Biotechnology | May 25, 2020
This publication provides an overview of the state of genetic biocontrol, focusing on several approaches that were the subject of presentations at the Genetic Biocontrol for Invasive Species Workshop, which was sponsored by the OECD’s Co-operative Research Program on Biological Resource Management for Sustainable Agricultural Systems.
Agricultural Biotechnology and Biosafety in South Asia: Progress and Prospects
SAARC Agriculture Centre | September 30, 2019
This book contains the papers and proceedings of the SAARC Regional Consultative Meeting on the Progress and Prospects of Agricultural Biotechnology and Biosafety in South Asia, which took place on June 18- 20, 2019 in Dhaka, Bangladesh.
Problem Formulation for Gene Drive Mosquitoes Designed to Reduce Malaria Transmission in Africa: Results from Four Regional Consultations 2016–2018
Malaria Journal | October 15, 2019
This summary publication captures the findings from four African consultations organized by the New Partnership for Africa’s Development (NEPAD) to identify risk hypotheses and data needs for future environmental risk assessment of gene drives in the mosquito Anopheles gambiae.
OECD Consensus Document of the Biology of Mosquito Aedes aegypti
Safety Assessment of Transgenic Organisms in the Environment (Volume 8) | June 23, 2018
Volume 8 of the series Safety Assessment of Transgenic Organisms in the Environment contains the first OECD biosafety consensus document to deal with the biology of an insect, the mosquito Aedes aegypti.
Capacities for the Risk Assessment of GMOs: Challenges to Build Sustainable Systems
Frontiers in Bioengineering and Biotechnology | April 5, 2018
The need for functional risk assessment bodies in general, and in the biosafety field in particular, demands continued efforts and commitment from regulatory agencies, if results that are sustainable in time are to be achieved. Dr. Carmen Vicién, Agriculture & Food Systems Institute in-country partner, was a co-author of this paper, which references the Agriculture & Food Systems Institute’s involvement in the Partnership for Biosafety Risk Assessment and Regulation in Paraguay and the use of Agriculture & Food Systems Institute eLearning courses in Kenya.
Events
-
 Environmental Risk Assessment Workshop: Non-Target Organism Testing
Environmental Risk Assessment Workshop: Non-Target Organism TestingJune 23, 2025-June 27, 2025
St. Louis, Missouri, USA
-
 OECD Working Party on the Harmonisation of Regulatory Oversight in Biotechnology (WP-HROB)
OECD Working Party on the Harmonisation of Regulatory Oversight in Biotechnology (WP-HROB)March 24, 2025-March 26, 2025
Paris, France
-
 Environmental Risk Assessment Workshop: Non-Target Organism Testing
Environmental Risk Assessment Workshop: Non-Target Organism TestingJune 24, 2024-June 28, 2024
Ames, Iowa, USA
-
 OECD Working Party on the Harmonisation of Regulatory Oversight in Biotechnology (WP-HROB)
OECD Working Party on the Harmonisation of Regulatory Oversight in Biotechnology (WP-HROB)March 18, 2024-March 20, 2024
Paris, France