The Issue
Developing and applying sound science to the environmental risk assessment (ERA) of biotechnologies is crucial for safely realizing their contributions to human health and sustainable production of food, fuel, and fiber.
What We Are Doing
Whether it be through improving systematic approaches to inform understanding of plausible risks associated with the use of gene drive strategies or working to maximize the utility of confined field trial data, we serve as a scientific resource for governments, academic institutions, and private sector organizations.
Why It’s Important
Any new technology comes with potential benefits and potential risks for the environment. Understanding these is essential to making informed decisions about what should or should not move from research to field to market.
Program Staff

Andrew Roberts, Ph.D.

Karen Hokanson, Ph.D.
Collaborators & Partners
- Association for Strengthening Agricultural Research in Eastern and Central Africa (ASARECA)
- Australian Government
- Common Market for Eastern and Southern Africa (COMESA)
- Foundation for the National Institutes of Health (FNIH)
- International Food Policy Research Institute (IFPRI)
- Instituto Interamericano de Cooperación para la Agricultura (IICA)
- New Partnership for African Development (NEPAD)
- Organisation for Economic Co-operation and Development (OECD)
- University of British Columbia
- University of Edinburgh
- USDA Foreign Agricultural Service
Programs

Promoting Use of Relevant Field Trial Data Across Geographies
Confined field trials (CFTs) are conducted to inform environmental risk assessments of genetically engineered crops. But instead of repeating trials in every country where a genetically engineered crop might be grown, data from previous trials, including those cultivated in similar agroclimates, may provide all that is necessary.
LEARN MORE
Rationalizing the Location of CFTs to Maximize Data Utility
Our online tool allows users to visualize agroclimatic zonations and their relationships to CFT locations in order to help determine where to best locate CFTs and maximize data utility. This can be used by CFT managers and risk assessors to provide context for the relevance of CFT data produced at remote locations.
VISIT TOOL
Identifying Priorities for Risk Assessment
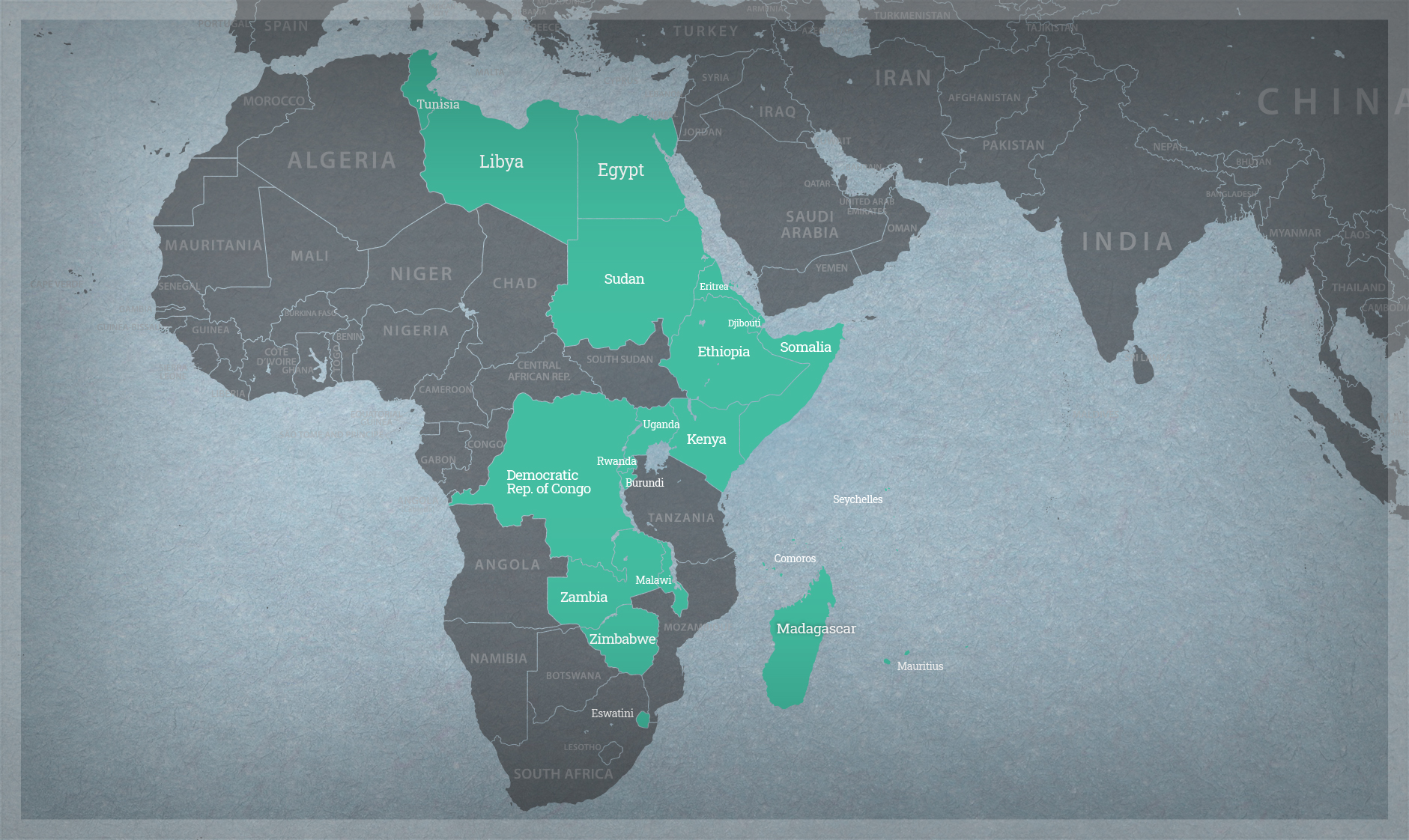
With 228 million cases of malaria worldwide in 2018, novel approaches to managing this debilitating disease are needed. We are advancing understanding of the underlying technology and helping stakeholders identify priorities for risk assessment of the use of gene drive to control the mosquito vectors that spread this disease.
LEARN MOREOur Successes
Because it is not possible to measure, monitor, and test every organism in every environment, surrogate species play an important role in evaluating the potential for adverse environmental impacts of new technologies. This includes assessing the potential effects of genetically engineered crops on non-target organisms (NTOs). Surrogate species must be selected appropriately to represent key taxonomic or functional groups and testing must be done with sufficient rigor to ensure that tests address the potential impact being investigated.
- The Agriculture & Food Systems Institute facilitated expert working groups, training workshops, and publications addressing the proper use of surrogate species for testing in support of environmental risk assessment of genetically engineered crops.
- In 2016, the Agriculture & Food Systems Institute produced a peer-reviewed publication that provides an overview of the history of regulatory approaches to NTO assessments and analyzes current regulatory practices. The paper proposes recommendations to improve the predictive value and efficiency of NTO testing, as well as facilitating the transportability of existing datasets across geographies and regulatory regimes.
Genetically engineered Ae. aegypti have been developed to control populations of this mosquito, which is the main vector of yellow fever, dengue fever, Zika, and chikungunya. In order to facilitate risk assessments for the use of these GE mosquitoes, regulators need access to succinct, summary information of biosafety-relevant aspects of the biology of the species.
The OECD Working Group on Harmonization of Regulatory Oversight in Biotechnology (WGHROB) develops and publishes consensus documents that provide internationally agreed upon baseline information for understanding key aspects about the biology of specific organisms. Each document identifies and describes important characteristics of its subject, usually a plant species, which are typically considered when undertaking a comparative risk assessment of its genetically engineered counterpart.
The Agriculture & Food Systems Institute approached the OECD Environment Secretariat about developing a biology document for Aedes aegypti, the first insect species and only the second animal species to be the subject of an OECD biology document. The idea was put to the WGHROB and agreed, with the Governments of Brazil and Mexico as convening leads together with the Agriculture & Food Systems Institute. The OECD Consensus Document on the Biology of Mosquito Aedes aegypti was declassified in July 2018 and is now available on the OECD website.
RNA interference, or RNAi, refers to a set of biological processes that make use of conserved cellular machinery to silence genes. The use of RNAi to develop plants with desirable traits has fueled a discussion about how best to address environmental considerations that might arise with these products.
The Agriculture & Food Systems Institute convened a multi-sectoral, multi-disciplinary team of scientists to help identify and clarify the relevant safety considerations and risk assessment methodologies for environmental risk assessment of plants with RNAi traits.
- This resulted in the publication of a peer-reviewed paper that identifies research informative for environmental risk assessments of these plants, with a particular focus on non-target organism assessment.
- This important work was highlighted during scientific sessions at the 2012 Entomological Society of America Annual Meeting and the 13th International Symposium for the Biosafety of Genetically Modified Organisms.
Discover
Publications
Frequently Asked Questions: Genome Edited Plants
Biosafety Resource Book Series | April 4, 2024
Published by the South Asia Biosafety Program, this booklet provides information about genome edited plants in a simple language, so readers may better understand the science, applications, and policies at the global and national levels.
GEnZ Explorer: A Tool for Visualizing Agroclimate to Inform Research and Regulatory Risk Assessment
Transgenic Research | June 6, 2023
This paper describes the development of an open-source tool, the GEnZ Explorer, that provides agroclimate together with overall crop production information to assist regulators and applicants in making informed choices on whether data from previous CFTs can inform an environmental risk assessment in a new country, as well as help developers determine optimal locations for planning future CFTs. The GEnZ Explorer allows users to identify the agroclimate zones that are relevant for the production of 21 major crops and crop categories.
New Applications of Insecticidal RNAi
Frontiers in Agronomy | May 27, 2022
Reduced reliance on synthetic chemical pesticides that may negatively impact pollinators and other beneficial insects is an aim of both private industry and governmental agencies alike. The need for environmentally sound methods of insect control has inspired research into a wide range of bio-based technologies. RNA interference has emerged as a highly effective tool with inherent potential to control a wide range of insects.
Frequently Asked Questions: Genetically Engineered Plants and Biosafety
Biosafety Resource Book Series | March 30, 2021
The South Asia Biosafety Program designed this booklet to address some of the questions the general public may have about the safety of genetically engineered plants, with explanations in easy and understandable language.
Biosafety Regulation and Processes in Bangladesh: A Guide for Researchers in Agricultural Biotechnology
Biosafety Resource Book Series | November 19, 2020
Part of the South Asia Biosafety Program’s capacity development interventions, this publication aims to inform researchers about the prevailing regulatory administrative system of Bangladesh and outlines the regulatory processes functioning at different stages of research and development of GE crops.
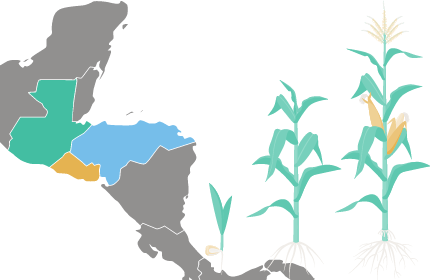
Paraguay’s Path Toward the Simplification of Procedures in the Approval of GE Crops
Frontiers in Bioengineering and Biotechnology | August 18, 2020
This paper presents the recent evolution of the regulatory system in Paraguay toward the establishment of a simplified procedure for GE crops that have been already assessed by sound and experienced regulatory systems, taking into account several scientific criteria. Dr. Carmen Vicién, Agriculture & Food Systems Institute in-country partner, was a co-author of this paper, which references the Agriculture & Food Systems Institute’s involvement in the Partnership for Biosafety Risk Assessment and Regulation in Paraguay.
Sublethal Endpoints in Non-Target Organism Testing for Insect-Active GE Crops
Frontiers in Bioengineering and Biotechnology | June 9, 2020
This review paper focuses on the current status and history of sublethal endpoint use in insect-active GE crops and evaluates the future use of sublethal endpoints for new and emerging technologies.
Genetic Biocontrol for Invasive Species
Frontiers in Bioengineering and Biotechnology | May 25, 2020
This publication provides an overview of the state of genetic biocontrol, focusing on several approaches that were the subject of presentations at the Genetic Biocontrol for Invasive Species Workshop, which was sponsored by the OECD’s Co-operative Research Program on Biological Resource Management for Sustainable Agricultural Systems.
Agricultural Biotechnology and Biosafety in South Asia: Progress and Prospects
SAARC Agriculture Centre | September 30, 2019
This book contains the papers and proceedings of the SAARC Regional Consultative Meeting on the Progress and Prospects of Agricultural Biotechnology and Biosafety in South Asia, which took place on June 18- 20, 2019 in Dhaka, Bangladesh.
Problem Formulation for Gene Drive Mosquitoes Designed to Reduce Malaria Transmission in Africa: Results from Four Regional Consultations 2016–2018
Malaria Journal | October 15, 2019
This summary publication captures the findings from four African consultations organized by the New Partnership for Africa’s Development (NEPAD) to identify risk hypotheses and data needs for future environmental risk assessment of gene drives in the mosquito Anopheles gambiae.
OECD Consensus Document of the Biology of Mosquito Aedes aegypti
Safety Assessment of Transgenic Organisms in the Environment (Volume 8) | June 23, 2018
Volume 8 of the series Safety Assessment of Transgenic Organisms in the Environment contains the first OECD biosafety consensus document to deal with the biology of an insect, the mosquito Aedes aegypti.
Capacities for the Risk Assessment of GMOs: Challenges to Build Sustainable Systems
Frontiers in Bioengineering and Biotechnology | April 5, 2018
The need for functional risk assessment bodies in general, and in the biosafety field in particular, demands continued efforts and commitment from regulatory agencies, if results that are sustainable in time are to be achieved. Dr. Carmen Vicién, Agriculture & Food Systems Institute in-country partner, was a co-author of this paper, which references the Agriculture & Food Systems Institute’s involvement in the Partnership for Biosafety Risk Assessment and Regulation in Paraguay and the use of Agriculture & Food Systems Institute eLearning courses in Kenya.
Events
-
 OECD Working Party on the Harmonisation of Regulatory Oversight in Biotechnology (WP-HROB)
OECD Working Party on the Harmonisation of Regulatory Oversight in Biotechnology (WP-HROB)March 18, 2024-March 20, 2024
Paris, France
-
 Workshop on Risk Assessment for Novel Insect Resistance Technologies in Genetically Engineered Plants
Workshop on Risk Assessment for Novel Insect Resistance Technologies in Genetically Engineered PlantsSeptember 14, 2023-September 15, 2023
Washington, DC
-
 Environmental Risk Assessment Workshop: Non-Target Organism Testing
Environmental Risk Assessment Workshop: Non-Target Organism TestingJune 26, 2023-June 30, 2023
Ames, Iowa, USA
-
 16th ISBR Symposium (ISBR 2023)
16th ISBR Symposium (ISBR 2023)April 30, 2023-May 4, 2023
St. Louis, MO, USA